A Breakthrough Approach
for Minimally-Invasive Hysterectomy
Designed to Improve
Safety, Speed & Simplicity
Hysterectomy is the second most common surgery for women1, yet many current approaches fall short:

High container breach rates risk hidden cancer spread or bowel injury2-6

Significant abdominal incisions leave scars and cause unnecessary hospital readmissions7
Procedure times are highly variable2
introducing
The Claria System
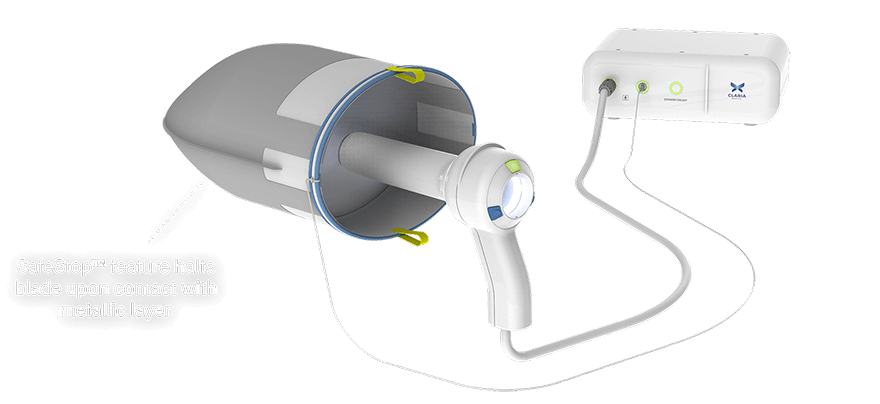
An intelligent system designed for safe extraction of an enlarged uterus using a vaginal approach with no mini-laparotomy, minimizing patient risk, recovery and scarring.
Safety
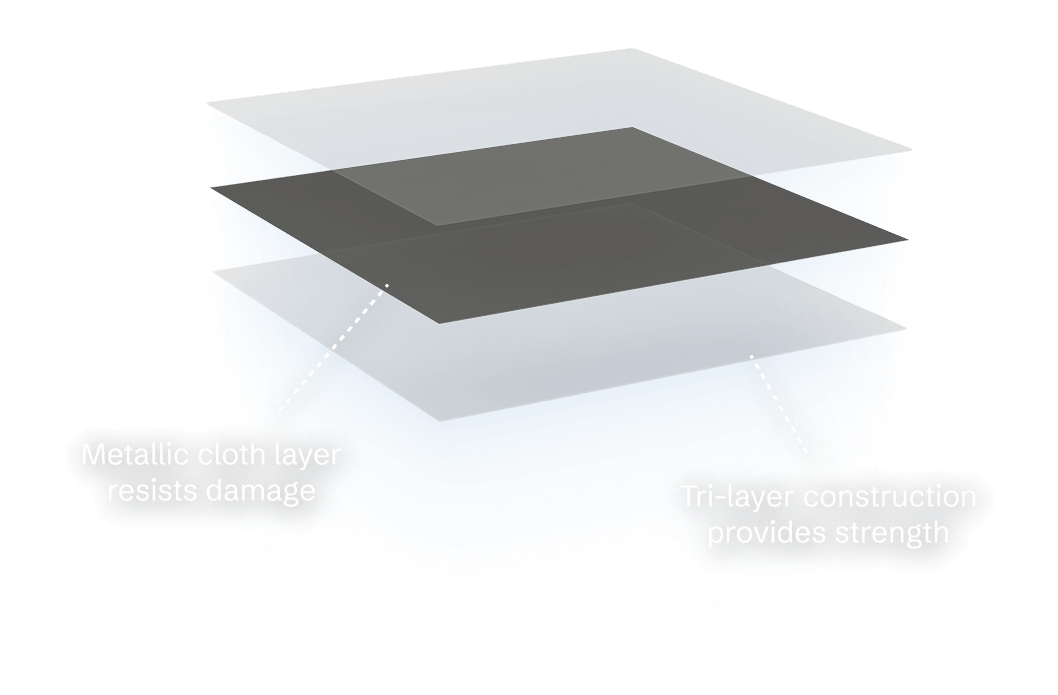
Redundant safety features make the Container breach-resistant to prevent unintended anatomical contact or accidents.
Ease of Use
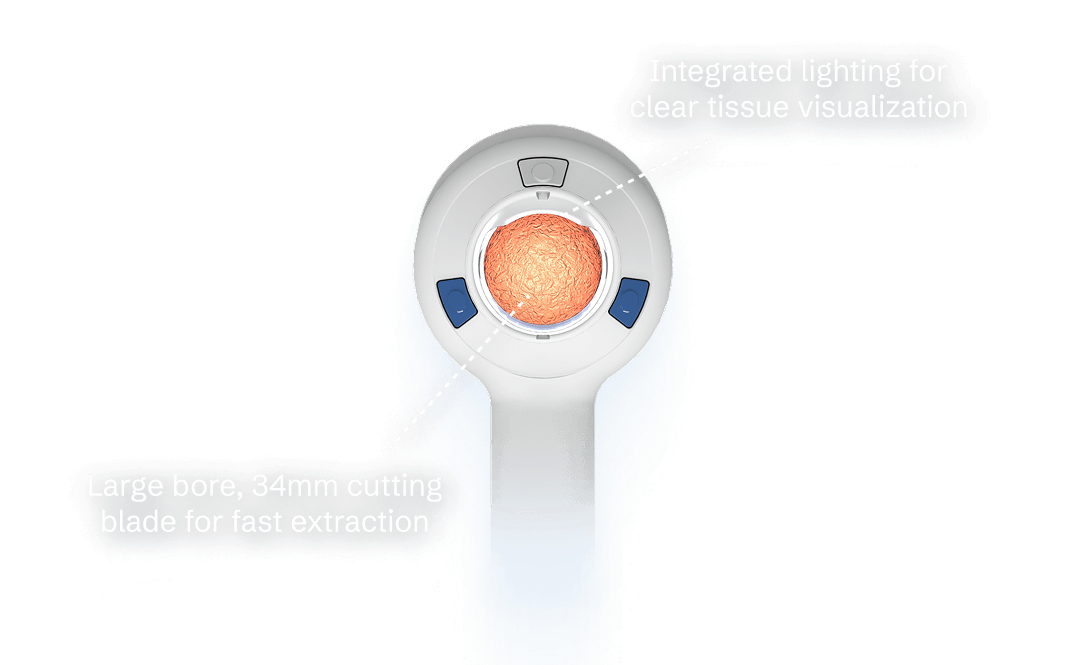
Integrated lighting and blade within the large-bore handpiece enables reliably rapid extraction of large tissue volumes.
Watch The Claria System in Action
Scientific Presentations
Claria Medical has been awarded prestigious National Science Foundation and National Institute of Health grants to develop products to improve hysterectomy, myomectomy and other surgical procedures.


- Wu JM, Wechter ME, Geller EJ, Nguyen TV, and Visco AG (2007). Hysterectomy rates in the United States, Obstetrics and Gynecology, 110:1091–5.
- Cohen SL, Clark NV, Ajao, MO, Brown DN, Gargiulo AR, Gu X, and Einarsson JI (2019). Prospective Evaluation of Manual Morcellation Techniques: Minilaporotomy versus Vaginal Approach. The Journal of Minimally Invasive Gynecology, 26:4, p. 702-708.
- Cohen SL, Morris SN, Brown NB, Greenberg JA, Walsh BW, Gargiulo AR, Isaacson KB, Wright KN, Srouji SS, Anchan RM, Vogell AB and Einarsson JI (2016). Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Obstetrics and Gynecology, 257e1-e6.
- Solima E, Scagnelli G, Austoni V, Natale A, Bertulessi C, Busacca M and Vignali M (2015) Vaginal Uterine Morcellation Within a Specimen Containment System: A Study of Bag Integrity. The Journal of Minimally Invasive Gynecology, Vol 22, No 7. p 1244-1246.
- Milad, M. P., & Milad, E. A. (2014). Laparoscopic morcellator-related complications. Journal of Minimally Invasive Gynecology, 21(3), 486–491.
- FDA Manufacturer and User Facility Device Experience (MAUDE) database, Search of Product Code: PMU, Date Range: 1/1/2014-5/31/2025
- Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. (2015). Underlying reasons associated with hospital readmission following surgery in the United States. Journal of the American Medical Association. 313(5):483-95.

